Abstract
Introduction: There is a significant need for improved therapeutics for older patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Five-year survival rates dip sharply as patient age increases, falling to ~40-45% for patients with ALL over 40 years of age and ~10% for adults with AML over 65 years of age. Cell surface expression of CD25 on AML and ALL blast cells is associated with adverse outcomes, including induction failure, relapse, and shorter overall survival. ADCT-301 (camidanlumab tesirine) is an antibody drug conjugate composed of a human CD25-targeting monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer cytotoxin. Camidanlumab tesirine (Cami-T) has demonstrated anti-tumor efficacy in mouse xenograft models of CD25-expressing hematologic malignancies. We present interim data from a Phase 1 study of Cami-T treatment in patients with relapsed or refractory (R/R) CD25-positive acute leukemia.
Methods: A Phase 1, open-label, dose-escalation (part 1) and expansion (part 2) multicenter, US study is enrolling patients with R/R CD25-positive acute leukemia. The primary objective is to assess the tolerability and safety of Cami-T and determine the maximum tolerated dose (MTD). Investigator-assessed anti-tumor activity, pharmacokinetics (PK), and the presence of anti-drug antibodies are also being evaluated. Eligible patients are ≥18 years of age, have CD25-positive AML or ALL without extramedullary disease, have failed or are intolerant to established therapy, and have an ECOG performance status of 0-2. CD25-positive AML or ALL is defined as CD25 expression on ≥5% of leukemic cells within bone marrow aspirate or biopsy. In Part 1, patients are assigned to treatment using a 3+3 dose-escalation design, based on assessment of dose-limiting toxicities (DLTs) during Cycle 1, to determine the MTD. Cami-T is given as an intravenous infusion on Day 1 of each cycle. The first cohort started treatment on a 21-day (q3w) cycle at a starting dose of 3 μg/kg. Dose frequency in subsequent cohorts may increase to once-weekly (qw) in the absence of DLTs. Part 2 will further evaluate safety, tolerability, PK and clinical activity at the dose recommended from Part 1.
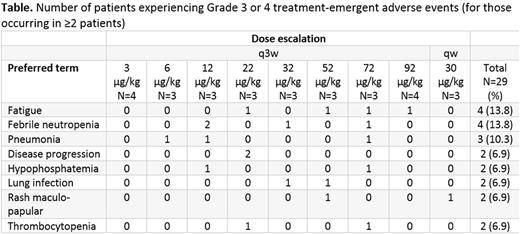
Results: As of June 26, 2017, 29 patients (20 male, 9 female) with AML have been treated with Cami-T (median 2 cycles, range: 1-7). The median age is 67 years [range 22-82] and patients had received a median of 3 (range: 1-9) previous chemotherapies. Five (17%) patients had undergone prior allogeneic stem cell transplantation. No DLTs were observed up to the highest evaluated q3w dose of 92 µg/kg. After switching to weekly dosing, one DLT was reported in the 30 μg/kg dose group. A total of 250 treatment-emergent adverse events (TEAEs) were reported in 28/29 (97%) patients. The most common TEAEs were fatigue and nausea (both n=7) followed by diarrhea, pneumonia and pyrexia (all n=5; Table). Grade ≥3 TEAEs were reported by 22/29 (76%) patients, of which fatigue (n=4), febrile neutropenia (n=4) and pneumonia (n=3) were the most common. Five deaths from TEAEs were recorded (one case each of blood disorder, cardiac arrest and pneumonia and two cases of disease progression). Two patients experienced infusion-related reactions. Three patients experienced TEAEs leading to a dose delay or reduction (one case each of skin rash, pericarditis and supraventricular tachycardia), and one patient each in the 52 μg/kg q3w and 30 μg/kg qw dose groups discontinued treatment due to Grade 3 skin rash. Transient CD25+ blast clearance in 2 patients who received 1 and 7 cycles, respectively, of Cami-T 32 μg/kg q3w, was observed, supporting an on-target activity of Cami-T. No responses or remissions have been obtained to date. PK data show increasing Cami-T total and PBD-conjugated antibody exposure with dose. Rapid systemic clearance of the drug with levels below limits of quantification suggest that q3w dosing may be insufficient for therapeutic efficacy, prompting exploration of a qw schedule.
Conclusions:
In this ongoing Phase 1 study in patients with CD25-positive R/R AML, single-agent Cami-T has shown an acceptable safety profile thus far. Additional patients with AML and ALL are being enrolled and dose escalation will continue until the MTD is identified, with further results expected later this year.
Study sponsored by ADC Therapeutics. https://clinicaltrials.gov/ct2/show/NCT02588092
Goldberg: Pfizer: Research Funding; ADC Therapeutics: Research Funding; Genentech: Research Funding. Solh: ADC Therapeutics: Research Funding; Amgen: Speakers Bureau; Celgene: Speakers Bureau. Ungar: ADC Therapeutics: Employment, Other: Stock option interest. Rizzieri: Erytech: Research Funding; Shire: Research Funding. Walter: ADC Therapeutics: Research Funding; Aptevo Therapeutics: Research Funding. Spira: ADC Therapeutics: Research Funding. Chung: ADC Therapeutics: Research Funding. Stock: Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy. He: ADC Therapeutics: Employment, Other: Potential equity interest. Boni: ADC Therapeutics: Employment, Other: Potential equity interest. Atallah: ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal